Product introduction
Nipah Virus (NIV) Nucleic Acid Detection Kit (RT-qPCR, lyophilized type)
Nipah Virus (NIV) Nucleic Acid Detection Kit (RT-qPCR, lyophilized type)
Nipah Virus (NIV) Nucleic Acid Detection Kit (RT-qPCR, lyophilized type) is based on quantitative real-time PCR technology. It utilizes specific primers and probes designed for the highly conserved nucleocapsid protein N gene of the Nipah virus. Using the TaqMan probe method, it monitors the PCR amplification process in real time, allowing for accurate identification of Nipah virus nucleic acid in samples and enabling qualitative detection of the virus.
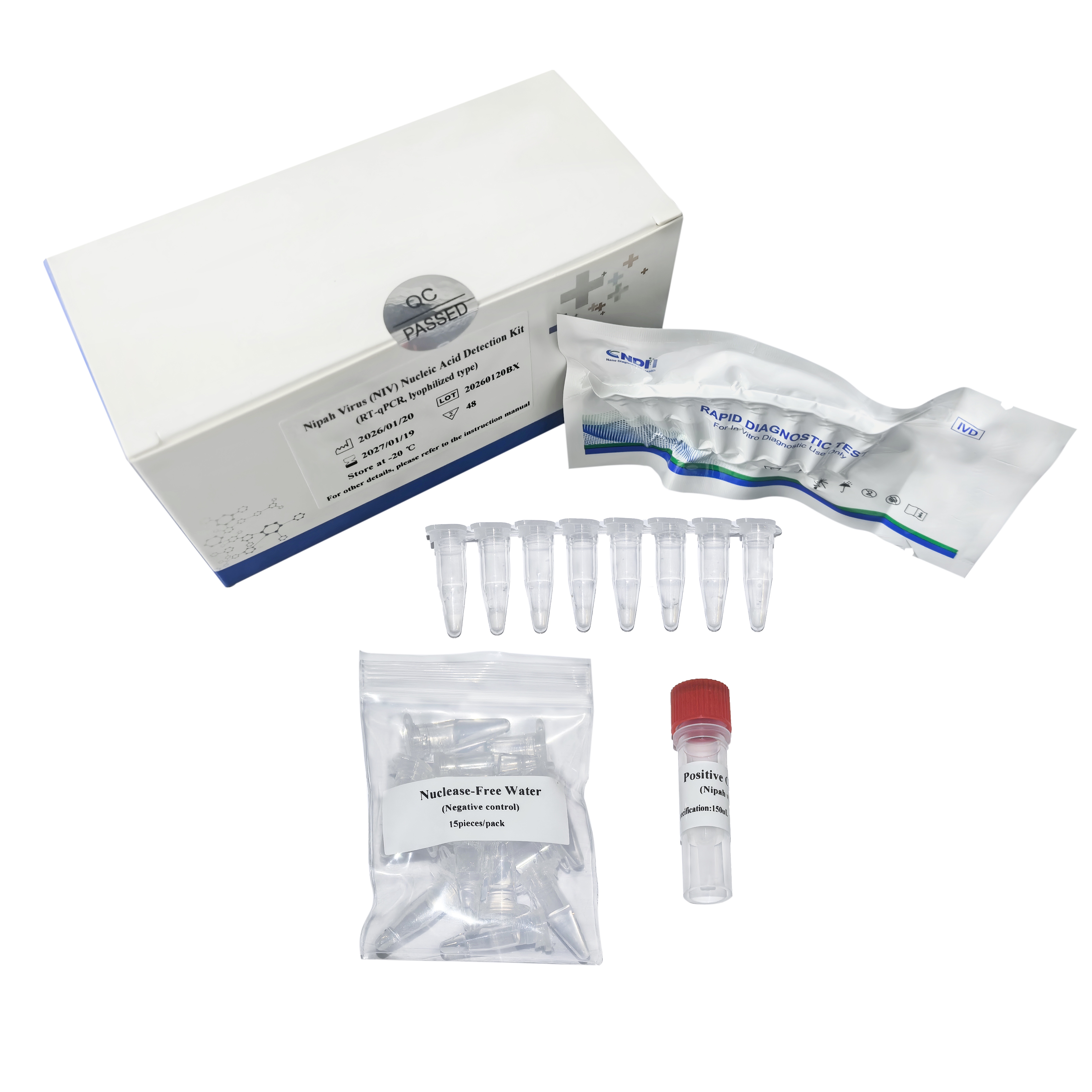
2. Using lyophiliz technology, convenient for transportation and storage;
3. Utilizes integrated pre-mixed reagents for immediate testing upon adding sample;
4. Includes endogenous internal controls for effective quality control throughout sample collection and laboratory testing.
5. Compatible with various models of real-time qPCR machines.
Technical Information
| Pathogen | Nipah Virus (NIV) |
| Methodology | Fluorescent PCR |
| LOD | 20 copies/ml |
| Specimen type | Serum, plasma, whole blood, cerebrospinal fluid, throat swab, secretions, tissue homogenate, urine, virus culture |
| Test time | ≤70min |
| Internal control | Endogenous internal control |
| CV% | ≤5% |
| Pack Spec. | 48 tests / kit |
| Storage condition & Shelf life |
Store at -20℃,shelf life: 12 months
For short-term use, it can be stored at 4℃ for no more than 1 month.
|
Nipah Virus Monitoring Solution
Contact us for more product information
Nano Diagnosis for Health Biotech (Guangzhou) Co., Ltd
Address: 7F&8F, D Bldg. No. 136 Kaiyuan Avenue, Huangpu District,
Guangzhou, China 510535
Tel: +86 20 28399556
Email: sales@cndhbio.com

WeChat official account
Copyright @ Nano Diagnosis for Health Biotech (Guangzhou) Co., Ltd
粤ICP备2022021939号-2